Automate Standard of Care Research. Save Millions.
The only provider of accurate, real-time SoC insights - pulled directly from global reimbursement authorities and delivered with a click.
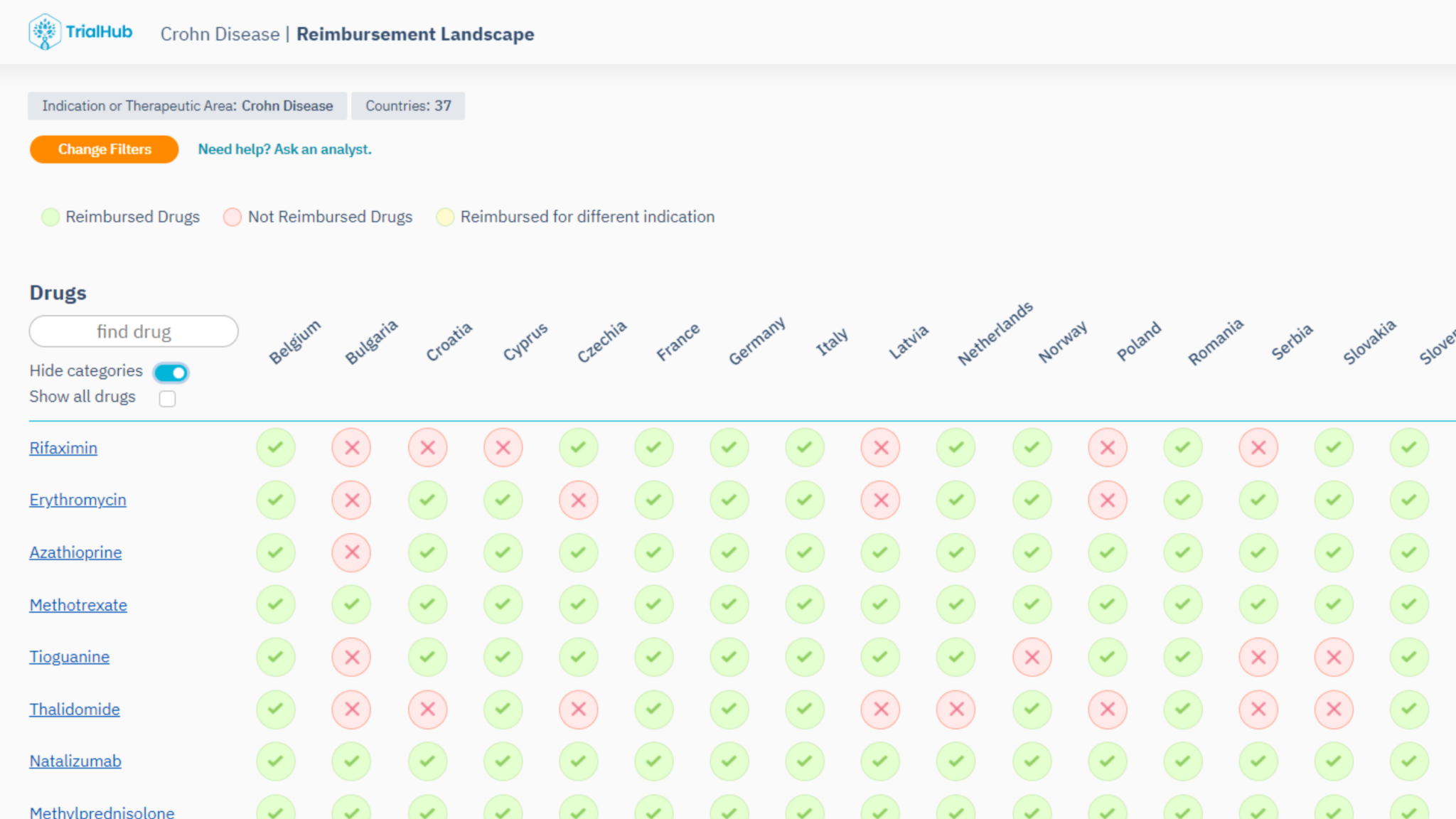
Standard Of Care: Your Global Solution to Patient-First Trial Planning
Explore patient access to treatments worldwide and prepare strategically for recruitment and retention challenges.
Trusted by the Clinical Research Leaders









Trusted by the Clinical Research Leaders





Stop the Cycle of Trial Delays and Costly Amendments

TrialHub Changes the Game
With automated global Standard of Care insights that cover:
Stop the Cycle
of Trial Delays
and Costly Amendments

TrialHub
Changes the Game
With automated global
Standard of Care insights that cover:
Get Started With These Use Cases
Attract Qualified Patients
Utilize our Patient Pathway Map for precise, up-to-date insights on treatment stages and locations to engage the right patients for your trial efficiently.
Understand Your Patient Population
Fully understand your target demographic in one click: reimbursement, treatment lines, dosage, drug brands, and pricing.
Avoid Costly Amendments
Rely on definitive reimbursement data from country regulatory bodies, not just HTAs, to align your trial with the local standard of care and prevent expensive amendments.
Save Time On Manual Research
Shift from 40 hours to 20 minutes: TrialHub delivers detailed, real-time data across any market, globally, including regional specifics. Speeding up your research dramatically.
Watch: Syneos Health Reduced 20x Time
on Reimbursement Research
Speed Up Recruitment
Utilize real-time enrolment trends and landscape insights
to optimize your recruitment strategy and efficiency. Еnsure patient retention with the most up-to-date data.
Read: Indegene Accelerates Patient Recruitment
For a Phase 3 Drug Trial of a Global Biopharma
Get Started
With These Use Cases
Attract Qualified Patients
Utilize our Patient Pathway Map for precise, up-to-date insights on treatment stages and locations to engage the right patients for your trial efficiently.
Understand Your Patient Population
Fully understand your target demographic in one click: reimbursement, treatment lines, dosage, drug brands, and pricing.
Avoid Costly Amendments
Rely on definitive reimbursement data from country regulatory bodies, not just HTAs, to align your trial with the local standard of care and prevent expensive amendments.
Save Time On Manual Research
Shift from 40 hours to 20 minutes: TrialHub delivers detailed, real-time data across any market, globally, including regional specifics. Speeding up your research dramatically.
Watch: Syneos Health Reduced 20x Time
on Reimbursement Research
Speed Up Recruitment
Utilize real-time enrolment trends and landscape insights to optimize your recruitment strategy and efficiency.
Read: Indegene Accelerates Patient Recruitment For a Phase 3 Drug Trial of a Global Biopharma
This is what TrialHub clients say:
Time is Your Ally
What used to take 40+ hours to uncover manually
is now accessible instantly with TrialHub.



This is what our TrialHub clients say
Time is Your Ally
What used to take 40+ hours to uncover manually is now accessible instantly with TrialHub.



Patient Centricity is no longer a myth.
Start understanding your patients’ journey with a 20-minute demo.
Patient Centricity
is no longer a myth.
Start understanding your patients’
journey with a 20-minute demo.

Join our cause:
Bringing Research Closer to Patients
TrialHub is part of FindMeCure Inc., a company dedicated to bringing Research closer to Patients:
- TrialHub is the first feasibility intelligence platform including patient feasibility – a new way to assess your protocol and trial strategy from the patients perspective.
- For each TrialHub client we donate a scholarship to a new patient advocate through the European Patients Academy.
- FindMeCure.com is the biggest free platfrom with clinical trials allowing patients to volunteer for clinical trials. Since 2016, we supported more than 4M people across the Globe.

Join our cause:
Bringing Research Closer to Patients
TrialHub is part of FindMeCure Inc.,
a company dedicated to bringing Research closer to Patients:
- TrialHub is the first feasibility intelligence platform including patient feasibility – a new way to assess your protocol and trial strategy from the patients perspective.
- For each TrialHub client we donate a scholarship to a new patient advocate through the European Patients Academy.
- FindMeCure.com is the biggest free platfrom with clinical trials allowing patients to volunteer for clinical trials. Since 2016, we supported more than 4M people across the Globe.
