Design the trials that patients deserve
And save millions of dollars on amendments
and delays.
Understand your patients and tap into their needs. With patient feasibility.
To design patient-aligned trials, you need to understand:
 Your patients’ disease burden and unmet healthcare needs.
Your patients’ disease burden and unmet healthcare needs. Your patients’ treatment pathway and Standard of Care.
Your patients’ treatment pathway and Standard of Care. Where your trial fits into their journey and what the burden of participation is for the patients.
Where your trial fits into their journey and what the burden of participation is for the patients.
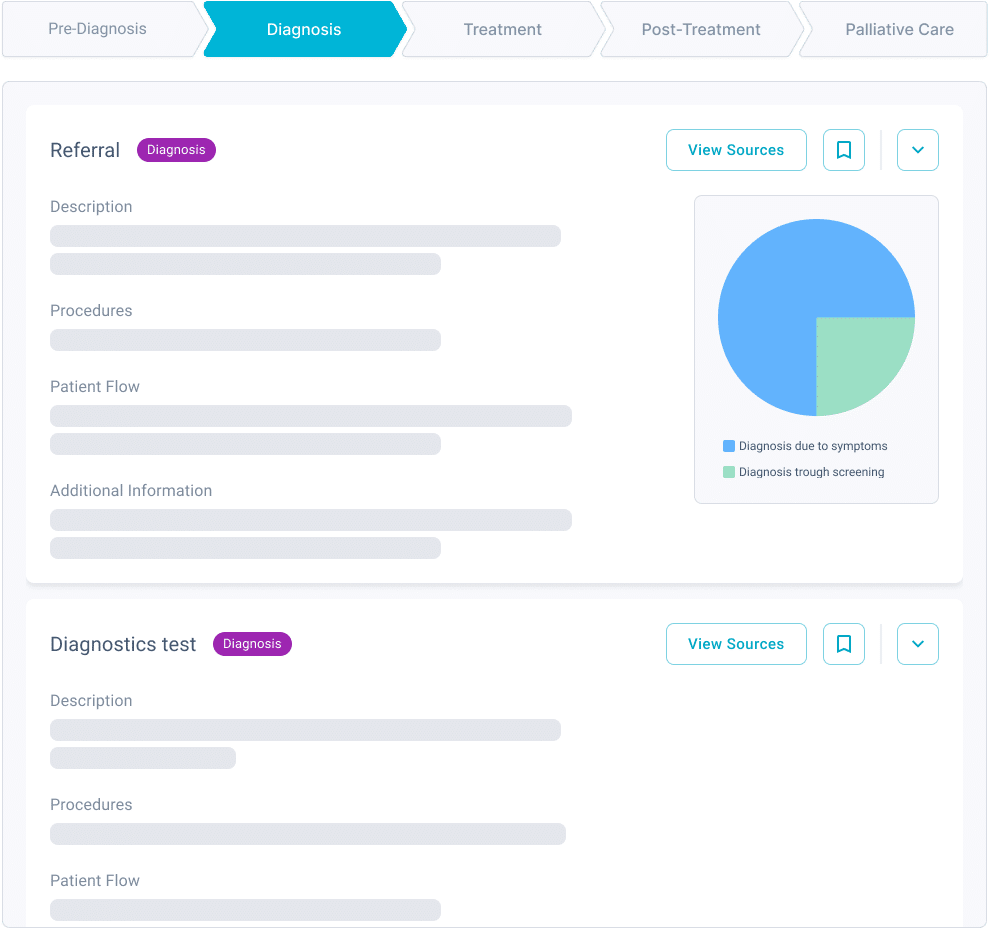
In short, you need to run a patient feasibility assessment.

Patient feasibility can reduce amendments and patient recruitment delays at least by half
 Identify where your eligible patients are.
Identify where your eligible patients are. Understand their SoC and access to treatments.
Understand their SoC and access to treatments. Tap into their unmet needs.
Tap into their unmet needs.
“Standard of care speaks to the eligibility of a patient. If a SoC drug renders a patient ineligible to participate in the study, that’s going to impact our recruitment and timelines.”

Annie Rotten
Manager, Strategic Site Solutions, Avance Clinical
Develop protocols that work in the real world
 Design realistic, practical trial protocols that align with local treatment practices.
Design realistic, practical trial protocols that align with local treatment practices. Ensure control arms accurately represent existing reimbursed treatments based on Standard of Care benchmarks.
Ensure control arms accurately represent existing reimbursed treatments based on Standard of Care benchmarks.
Validate the need for your treatment
 Compare your therapy against regional treatment standards to make sure you’re addressing unmet patient needs.
Compare your therapy against regional treatment standards to make sure you’re addressing unmet patient needs. Predict commercial viability and the potential impact of your therapy.
Predict commercial viability and the potential impact of your therapy. Enhance your product development strategy with accurate, data-driven insights for a better market position.
Enhance your product development strategy with accurate, data-driven insights for a better market position.
Identify the regions
with the highest
patient availability
 Estimate the potential number of patients who need your treatment in each country you are considering.
Estimate the potential number of patients who need your treatment in each country you are considering.
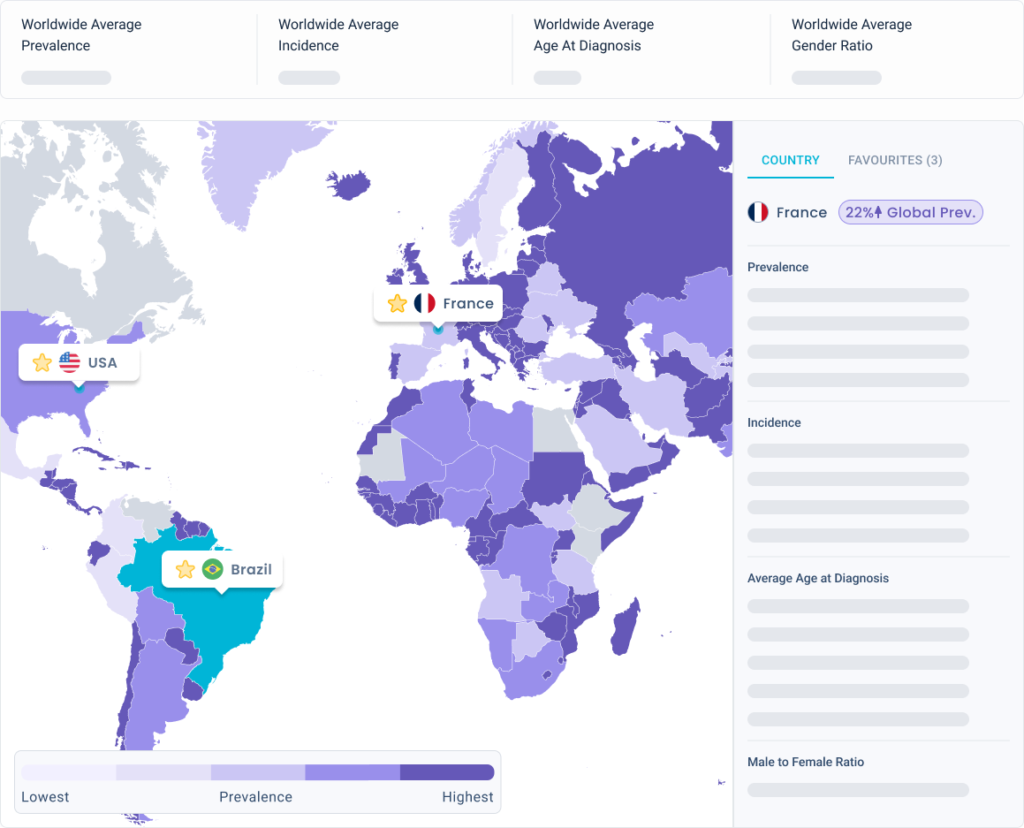
Identify countries with more eligible patients
 Know how patients are treated locally to spot potential recruitment challenges.
Know how patients are treated locally to spot potential recruitment challenges. If the Standard of Care makes patients ineligible, enrolling in that country will be challenging.
If the Standard of Care makes patients ineligible, enrolling in that country will be challenging.
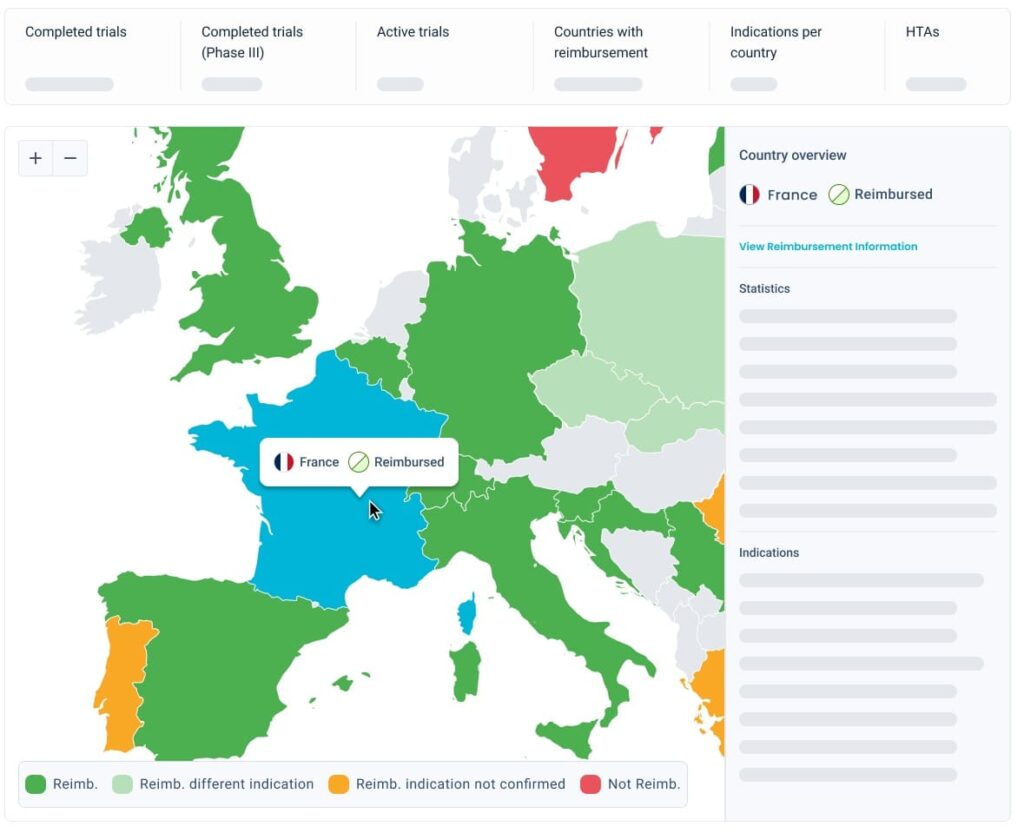
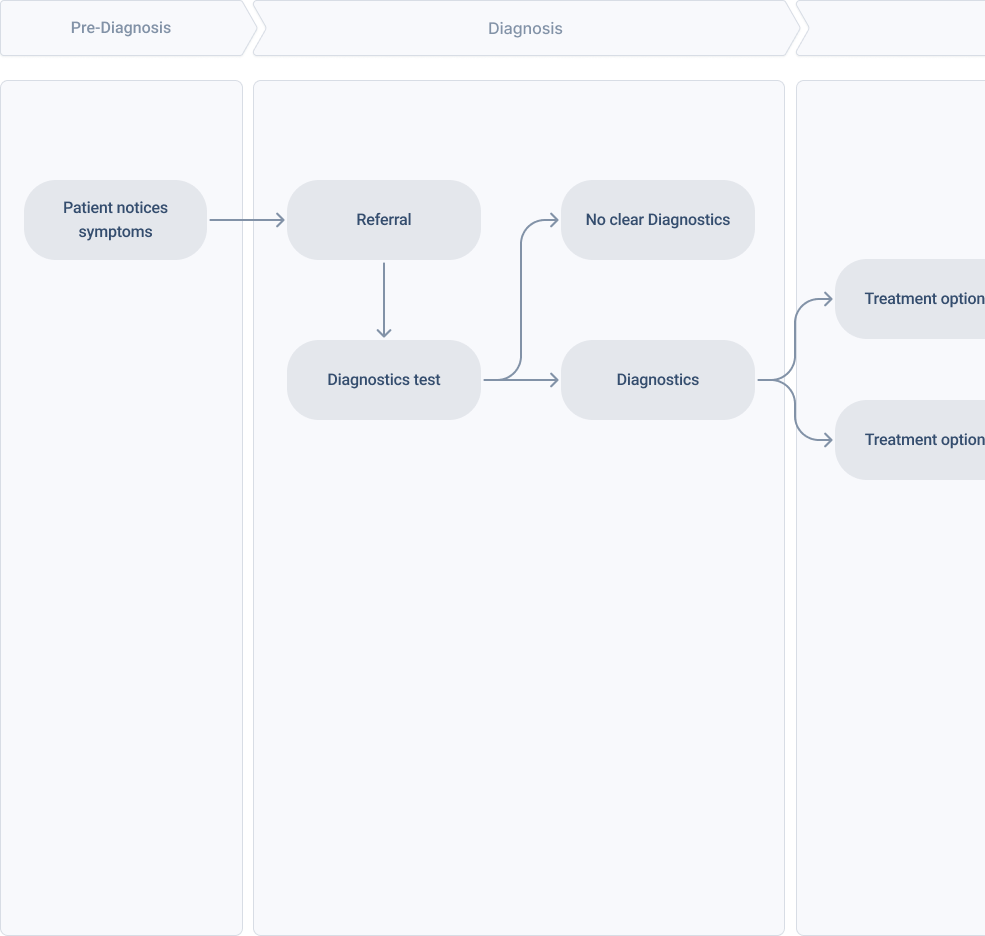
Understand the patient journey country by country
From diagnosis to treatment and disease burden, gain insight into your patient cohort so you can answer the questions:
 Where does my trial fit into their journey?
Where does my trial fit into their journey? At what stage do patients become eligible?
At what stage do patients become eligible? What are the unmet needs my trial can address?
What are the unmet needs my trial can address? How can I make it easy for patients to stay in my trial?
How can I make it easy for patients to stay in my trial?
We helped Boehringer Ingelheim turn patient insights into actionable data.
Here’s what they said about our partnership:
TrialHub enables a rapid translation of expansive datasets into actionable patient-centric insights, reducing the time needed for analysis from 3-6 months to several hours.
Tailored
to your needs
Discover what TrialHub can do for your team.
Pharma
Save millions of dollars on avoidable amendments.
Predict and prevent recruitment challenges before they arise.
Develop protocols that work in the real world.
CRO
Create a winning proposal 50% faster.
Design a trial strategy that earns the sponsor’s trust.
Monitor the ever-changing SoC landscape to predict and prevent recruitment delays.
Biotech
Validate your product and take your trial where it’s needed.
Select the right strategy for your study.
Monitor the dynamic clinical landscape to mitigate risks.
TrialHub is so intuitive to you, you’ll navigate it effortlessly. But we offer the best support you’ve received anyway.
With Ask the Analyst, TrialHub’s team becomes your team.
 Dedicated team of medical experts, analysts and data scientists just one click away.
Dedicated team of medical experts, analysts and data scientists just one click away. Exceptional, human customer support at every step of the way.
Exceptional, human customer support at every step of the way. A product roadmap developed with your needs in mind.
A product roadmap developed with your needs in mind.
We’re here to help your product reach patients faster
Don’t waste years in delays. Patients need innovative treatments now.