We are releasing a major update to our clinical trial feasibility platform TrialHub and we want to introduce you to the changes that will make the job of feasibility and patient recruitment specialists easier.
TrialHub 2.0 provides not only enhanced user experience but also double the clinical trials in our database and even more data on Sites & Investigators. As always, these updates were inspired by the feedback of industry professionals – our mission is to stay close to your needs and support you in the best way possible. You can reach out to our team inside the platform as you go about the planning of your trial – check it out.
TrialHub 2.0
Because this is a big update to TrialHub we decided to interview our dev team about the changes so you can get all of this information straight from the people behind the algorithms. If you’re curious about the technical details around data sources, data extraction, and how TrialHub automates RR (recruitment rate) calculations you can watch the whole interview here.
https://www.youtube.com/watch?v=hF-Uyq_jjBE
Now let’s take a look at the changes you’ll see on TrialHub.
New data sources – The WHO Clinical Trial Registry
TrialHub 2.0 includes a new public clinical trial registry in its database – the WHO Registry – which doubles the number of trials you can see on our platform. The data providers for the International Clinical Trials Registry Platform include the Chinese and Japanese clinical trial registers, the EU clinical trial register, the Pan African Clinical Trial Registry, and 12 more, not counting ClinicalTrials.gov which we already rely on for most of our data.
As our developers explain in the interview above, CT.gov is currently the go-to for site-level data as it’s the most detailed register out of the 17 included in the WHO Registry. However, we wanted to present you with a more complete picture of the clinical trial landscape on a global scale – and as you know, many trials outside the scope of FDA regulations (such as European or Asian trials) are not registered on CT.gov.
This new addition will enrich your search results for a number of trials conducted in a given country and it will provide additional trials to analyze when comparing your protocol for timeline and recruitment predictions.
More Sites & Investigators details
To be able to provide you with the most accurate and up to date information about sites and investigators, TrialHub crawls the internet in real-time searching for investigator contacts on the webpages of the hospital and/or university where they work. Now our database will include even more details to help you choose the right investigator for your trial
- Date and outcome of last FDA inspection
We get the information about the last FDA inspection straight from the FDA database – here are some interesting details you can now access:
- FDA Inspections for 2019 – 528
- FDA Inspections for 2020 – 48 (only for the first Quarter of the year)
In 2019 the results of the inspections showed:
- 346 – No objectionable conditions or practices were found
- 181 – Objectionable conditions were found but the problems do not justify further regulatory action.
- 1 – Objectionable conditions were found and regulatory and/or administrative sanctions by FDA are indicated.
You can get even more detail than this on TrialHub – for example, if you already have a site in mind, now you can check when its last FDA inspection took place and what was the outcome.
Virtual clinical trials
Since the beginning of this pandemic, our team realized one thing – clinical research can’t go on unless it adopts virtual trial technologies to relieve the burden on patients and make for safe and seamless recruitment.
To do design your protocol in such a way, you need to identify similar trials in your countries of interest – now TrialHub does this for you. The algorithm identifies virtual and hybrid trials to help you predict recruitment timelines, comply with regulations, and partner with investigators who already have experience.
TrialHub supports the first-ever database of investigators who have participated in virtual clinical trials. This is part of our mission to align stakeholders and help the research world move toward patient-centricity.
More and more accurate search filters
When you’re looking for trials with similar protocol to yours, you want to get as close as possible to the specifics of your study in order to predict the timelines and recruitment. TrialHub makes it so instead of comparing your trial to one or two similar trials, you can scale your predictions by dozens if not hundreds of trials to get the most accurate results possible.
This is why we introduced changes to our filters to make your search as convenient and as accurate as it can be. Take a look at our new filters system:
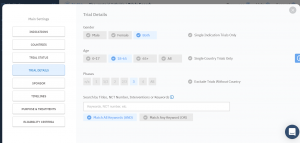
If you want to know more about the updates you can always reach out to our team. And remember that you can ask for support directly from inside the platform – just open the chat in the corner on the right.
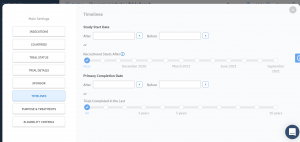
Step inside TrialHub to see what the future of feasibility looks like.

