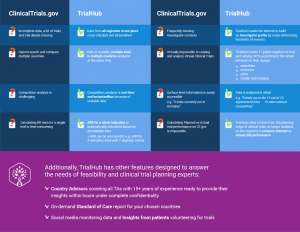
One of the points where data gets in the way of clinical research is in the very planning of a clinical trial. Feasibility experts often rely on public trial registers in order to choose the best countries according to their capacity, competitiveness, and patient population. However, when data there is incomplete it can be very misleading.
This is why TrialHub does not just get its data from ClinicalTrials.gov. We go the extra mile by collecting data in real-time from 17 public registers and thousands of other reliable sources and we combine this data with the real experience of local experts.
You’ll find other ways we improve and innovate on the most popular way of collecting feasibility data in the next infographic.