Patient recruitment in clinical research accounts for 32% of trial budgets – the largest single driver of clinical costs. Despite the heavy spending, it remains a huge challenge for the industry and the future of therapeutic innovation causing 50% of delays in clinical trials. Even so, many sponsors still rely on the traditional methods and approaches and bet on investigational sites to fulfill their enrollment targets and deadlines.
We have all heard about the negative statistics that two-thirds of sites don’t meet the enrollment requirements for a given trial (2011 Tufts Center for the Study of Drug Development (CSDD) report). 30% of them fail to recruit a single patient and such failures end up costing even more money for the industry, thus costing patients the chance to access better therapies sooner.
What was effective years ago may not be working as well today…or may not be working for all clinical trials. In the highly technological world we live in, it’s important for the drug development industry to also consider innovative tactics and opportunities, compare the outcomes and choose a new strategy.
What’s more, patients nowadays are increasingly more engaged in their own healthcare. They have access to tons of information, digital community forums and patient advocacy groups. They feel more empowered to take control of their own health. A lot of these patients are not in sites’ databases. This makes a direct-to-patient way of recruitment the obvious choice for certain trials. It includes using social marketing strategies, turning to new channels for interaction, referral programs, etc.
In this blog post, we explore the recent industry changes and trends, tips, challenges and mistakes to avoid in direct-to-patient recruitment.
What are the new trends in favour of Direct-to-Patient Recruitment?
- Data becomes more important
You no longer need to rely on a poster placed in the subway to attract the right people for your trial. The costs of standard methods of advertising cannot guarantee results. Platforms like Google and Facebook, however, can now target people in a specific city and people of a certain age. Ads are shown to those who have expressed interest in a certain condition or therapy and you can know upfront the potential outreach and how much this would cost.
- Increasing number of online patient databases
Many businesses have turned their direction towards creating digital patient communities divided by therapeutic area or a specific condition. By creating useful content and engaging patients through interactive materials and resources, they collect information about their behaviour, preferences and even their personal details. Such databases can be owned by a recruitment vendor or a medical website or health application and they can serve as a great source of potential clinical trial participants.
- The power of Patient Advocacy Groups
Patient communities have become a major source of information to patients looking for advice. Patient Advocacy Groups are becoming more active in uniting patients and providing them with personal help and support. They provide a platform where patients with similar journeys meet, connect and feel taken care of. Such organisations and support groups are more and more interested in research, provide education about the drug development process to their members and want to take an active role in clinical research. Patients who have been through clinical trials and are part of these communities, are becoming active ambassadors and serve as advocates of clinical trials.
What are the DO’s for Direct-to-Patient Recruitment?
- Use channels where you can target people specifically
Traditional advertising such as brochures, posters, TV and radio ads is related to huge costs, unpredictable results and the risk of missing valuable audiences. In the online world, you have many opportunities to target people specifically by condition, interest, gender or region. This helps you to narrow down your target audience and to spend your marketing budget wisely. You’re able to reach far more remote potential applicants, as well as more passive candidates. As mentioned above, platforms like Google, Facebook and Instagram can serve as a great source of potentially eligible and motivated patients.
Some other possibilities the digital way of recruitment gives you are mentioned below:
Google potential reach – you can do real-time data monitoring on people’s online search activity for a specific indication per country.Thus, you can get an estimation of the number of people that can be reached by indication-specific advertisement.
Advertising Competition – you can get real-time data on the level of advertisement for a specific indication (keywords). Such information allows you to allocate higher advertisement costs in order to reach a comparable number of patients.
Advertising restrictions – Understanding digital advertising potential for clinical trials requires in-depth knowledge of the regulatory landscape. As a rather novel advertising method, the requirements are fast-changing and not fully harmonised. Through TrialHub for example you can see most up-to-date information on country-specific legislation and country-specific requirements for different social media channels such as Facebook, Google, Instagram, etc.
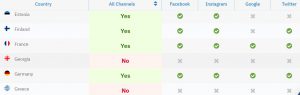
Clinical Trial Awareness – In many countries, the majority of people are poorly informed or even unaware of clinical trials. This can easily lead to incorrect assumptions and bias regarding the nature and intent of clinical studies. A low level of clinical trial awareness can hinder the success rate of patient recruitment. The digital recruitment approach allows you to do real-time data monitoring on people’s online search activity for clinical trials per country and their level of awareness.
Check TrialHub if you are interested for more information on these indicators.
- Focus on gathering data and patient insights to tailor your campaign
Recruitment plans start with understanding of the target patients. What is their journey – who diagnoses and treats them, what treatment options they have in their country, their unmet needs, daily obstacles and condition downfalls.
The best recruitment tactics will always differ depending on the type of population. Thus, it is important to develop a unique patient recruitment approach for each clinical trial. For example, if you are looking for patients for your Ocular or Generalized Myasthenia Gravis trial, consider patient-facing materials with bigger letters as such patients might have difficulty reading them. Or, if you are recruiting for a Diabetic foot trial, check whether patients are aware of this diagnosis and term or they simply know they have a wound on the foot/finger.
A good source of such insights are online patient communities and channels or patient organizations from the region/country. Speaking with such patients in advance and preparing better can make a huge difference for your campaign.
- Improve patients’ understanding about clinical trials
Like every human being, patients don’t like to be pushed to do something they don’t understand or wish to do. That is why it is important to educate your target audience about clinical trials and make them aware of the concept, all benefits, risks and things to consider before advertising anything specific to them. Only 15% of patients globally are aware that clinical trials are a treatment option and many people still have fears and concerns that need to be addressed properly.
Make sure you show empathy, walk in their shoes and show them you really care.
Write articles on the disease and what’s currently being researched, create information videos about clinical trials, share interactive infographics, interview patients who have been through this or/and doctors taking part in research. Let them ask you questions and support them during each step. Don’t simply advertise, but raise awareness, inform and educate.
- Track recruitment metrics and behaviour
Digital tools allow you to know all your recruitment funnel from ad to randomization. This approach gives you a possibility to compare predictions with results during and after the campaign so you can identify obstacles, take action and apply timely changes. Tracking and comparing results by channel, by type of audience and other parameters will help you spend your budget wisely, stay on top of your performance and be better prepared for future campaigns.
- Create partnerships with patient channels
Existing online databases such as digital health communities and mobile apps are potential partners that would help you reach the right people and inform them about the new research opportunity. Make sure you get to know their way of work, approach and business model and find a solution that works for both sides. It’s crucial to comply with the way they communicate with their audience and guarantee their rights to protect their users.
Also, collaborating with local, regional or national patient advocacy groups is always a good idea when it comes to knowing better your target patients and their journey. Patient organization representatives can give you invaluable insights on the big picture or specifics that are crucial for the message you are aiming to deliver. Please note, however, that not all organizations would be willing to help you with direct recruitment. Many patient advocates are still sceptical to promote clinical trials because of the fear of their patients being marketed or exposed to something specific that might not be relevant for them. You first need to check what their ethical code of conduct is and how they communicate with patients.
- Consider working with patient recruitment vendors
While you’ll need to invest in adopting a direct-to-patient recruitment strategy, the ROI is usually higher than traditional recruitment methods. How so? Well, when implemented effectively, you will meet enrollment targets in a shorter time frame. Simple.
Alternatively, you can consider working with patient recruitment vendors experienced in the field that have the necessary tools and know-how in place and can propose a variety of tailored strategies. Such companies have worked on various campaigns and are able to propose a solution based on your budget and expectations.
Different providers focus on different parts of the process so we are providing you with a list of companies and a summary of their services to help you decide which are the crucial aspects to you.
What are the DON’Ts for Direct-to-Patient Recruitment?
- Do not leave patients in the dark when ineligible
There are high chances that patients might turn ineligible when completing a pre-screener or when meeting the doctor at the screening visit. This is due to various factors such as eligibility criteria, complex clinical trial design or personal preferences of the patient. This, however, is not the moment where you as a company conducting research have to stop your engagement efforts. Going all the way through getting to know these people and their challenges, creating useful content for them, engaging them throughout the funnel until they get pre-screened/screened is a road that shouldn’t stop here – when you identify the patient’s ineligibility. The right next step is to show these patients all the other options in research they have. The patient isn’t looking for your clinical trial, they are looking for a solution to their health problem. Some guidance and advice on what they should do next would be enough for them. Show you care for the motivated person, not for the clinical trial only.
- Do not use highly scientific terms
Terms such as randomization, subjects, single-arm, double arm, double-blinded, adaptive pathway, phase1, phase 2, etc. are not something that a layperson would understand or find appealing. All of your messages and steps should be explained in a patient-friendly language and information should be presented in a way that is comprehensive enough for a person going through hard times. Patient-facing materials should show empathy and guidance, not medical excellence.
- Do not wait for the “rescue mode” to introduce changes
The beauty of the Direct-to-Patient Recruitment model is that it comes together with technology that allows you to be on track of all of your actions and their impact. As previously said, making data-backed predictions, identifying the key metrics and tracking the performance of your recruitment efforts can show you in real-time where the bottlenecks are and what can be improved. Make sure you introduce the necessary changes and test different scenarios before going into “rescue mode”. This will save costs and bring results much earlier. If problems come from an earlier stage, e.g the protocol design itself (60% of protocols need 1 or more amendments), then it’s crucial to get the decision-makers on board and discuss what could be done in a timely manner.
To conclude
No single industry can survive and sustain good results if decision-makers do not adapt to the current and future trends. Clinical research is no exception as now more than ever patients require special attention and approach. Although trials have not changed much in 70 years, everything else has. People are more connected than ever before and are more geographically distributed. Relying on traditional models would only freeze the potential for research to be conducted at an unprecedented scale and scope and bring innovative therapies sooner to those who need them.
Direct-to-Patient recruitment can be a great enhancement to your clinical trial but it cannot fix issues that occur on feasibility-level. Making sure your trial recruits enough patients to stay on budget on time begins with selecting the right sites and investigators not only for their experience but also for their proximity to eligible patients. TrialHub can help you with both of these challenges.
Sources:
https://www.reuters.com/article/idUS135923+22-Jun-2011+MW20110622
http://www.phrma.org/sites/default/files/pdf/2015_phrma_profile.pdf

