Prevalence, Past Experience, and Competition
Inflammatory bowel disease (IBD) is an umbrella term that describes multiple diseases that have in common chronic inflammation of the digestive tract. The most popular conditions included in the term are Ulcerative colitis and Crohn’s disease.
Currently, there is no cure for the debilitating condition which can lead to life-threatening complications. However, there are drugs being developed that show great potential to slow down the progress of IBD and provide symptom relief.
Prevalence
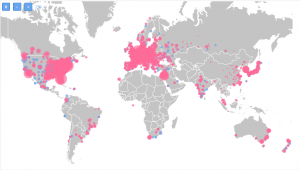
On the map below you can check the prevalence of IBD on a global scale.
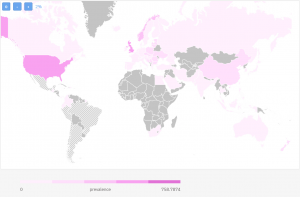
As you can see, IBD is mostly prevalent in the US and UK with Norway following behind.
Countries with Past Experience in IBD Clinical Research
The way we usually measure the research experience of a given country is by calculating the number of trials performed per one million people. The map below shows, on a global level, which countries have had more clinical trials than others based on their population:
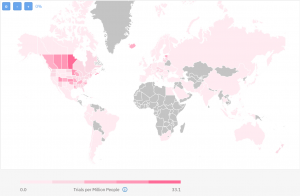
The darkest red regions are the ones with the most previous experience in running studies in IBD. Logically, IBD being most prevalent in the US and historically speaking Manitoba and Nova Scotia have the most completed trials per million people.
However, when we exclude Phase 4 and, if we only want to see trials conducted in the past 3 years, the map becomes more relevant to the current clinical research landscape:
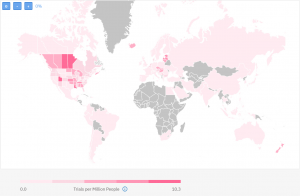
Utah, Louisiana, and Estonia and Latvia emerge as experienced destinations for clinical research in IBD, while Nova Scotia remains prolific with 10.3 completed trials per million people.
However, if we look at the number of trials completed in general (on a country level) divided by the number of people who live there (still filtering out phase 4 trials), then Latvia is leading the rankings, followed by other European countries:
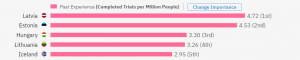
Interestingly, even if we remove the time limitations, Latvia still ranks among the first 3. Do you want to know which country ranks first? You can check on TrialHub.
Interesting Note:
Among the 2,058 analyzed trials, we identified 43 trials that had a virtual component included in their design. 12 of those research studies are Treatment focused. You can find their details on TrialHub.
Countries with Active or Recruiting Clinical Trials
At the moment there are 1,577 active, recruiting, or not yet recruiting clinical trials. Of them, the ones currently recruiting patients are 919.
The countries with the highest number of trials are the USA, Germany, Japan, and the Netherlands. However, if we want to better measure the competition level when it comes to patient recruitment, it’s best to measure trials versus population. Based on trials per million people, the top 5 countries with the highest competition are as follows:
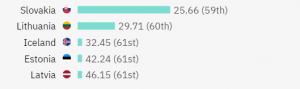
On the below map, you can also get a better idea of the global competition in running IBD clinical trials:
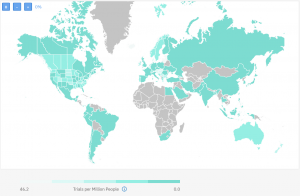
The dark green regions are the ones with fewer or no competitive trials.
As you can see on the map, there are regions with less competition even within Canada and the US where historically research has been focused. Also, some more prevalent regions like Norway currently have unoccupied sites which could be an opportunity for engaging their patient population.
To learn more about such opportunities – including the Patient Pathway in a given country, as well as Standard of Care – you can reach out to our team at patientsfirst@findmecure.com.
Recruitment Rates and Trials’ Performance Statistics
We have analyzed clinical trials for their Recruitment Rates and assessed countries based on their Average Recruitment Rate (patients/ site/ month). The map below represents the regions with high ARR in dark yellow, ranging between 2.67 patients/site/month and 0.02 patients per site per month.
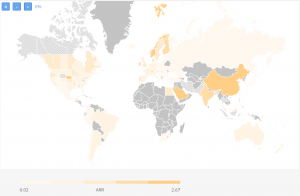
For Phase 3 studies only, ARR varies between 0.02 and 0.45 and for Phase 1 and 1/2 between 0.02 and 14.5 patients/site/month.
Looking at the highest recruiting countries, these are the top 5:

On the other end of the ranking, the countries that have the lowest ARR are:

All these rates are based on all sorts of research studies. If you’d like to know how the ranking changes for treatment-focused studies, you can log into TrialHub or get in touch with us to discuss and align your data needs with your protocol: patientsfirst@findmecure.com.
Interesting Stats:
Here are some statistics on the planned vs. actual performance of IBD clinical trials:

On average, IBD trials hugely overestimated the ARR which led to finishing with more sites than they started with and having to include rescue countries.
If you’d like to know more, you can check on TrialHub.
Sites and Investigators
At TrialHub, we have identified 7,190 research centers that have been working on clinical trials for IBD. You can see on the map how they are distributed. 6,110 of them have had at least one study focused on treatment.
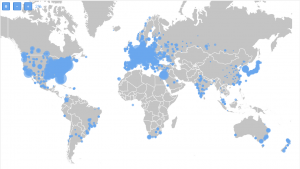
Highlighted in red are the ones currently working on trials in IBD:
When it comes to research sites that are currently not involved with studies in this indication, out of 7,190, we identified that 2,471 are currently not occupied.
Below, we provide you with a list of the most experienced organizations in IBD research in the countries with the highest number of trials per million people:
- Pauls Stradins Kliniska Universitates Slimnica, Latvia
- Tartu University Hospital, Estonia
- Pannonia Maganorvosi Centrum Kft., Hungary
There also are many investigators with backgrounds in IBD research. At TrialHub, we identified 7,401 such investigators. 5,206 of them have experience in trials focused on researching treatments. 455 of these investigators have worked on Phase 1 and Phase 1/2 studies.
Interesting Note:
The top 10 investigators with the most completed clinical trials in IBD are:
- Xavier Hebuterne – Nice, France
- Xavier Treton – Clichy, France
- Eric Lerebours – Rouen, France
- Jérome FILIPPI – Nice, France
- Yoram Bouhnik – Clichy, France
- Carmen STEFANESCU – Clichy, France
- Alain ATTAR – Clichy, France
- Robert Hardi – Chevy Chase, Maryland, United States
- Jean-Frédéric COLOMBEL – Lille, France
- Gwenola VERNIER-MASSOUILLE – Lille, France
To learn more about them, and other investigators available to run clinical trials in IBD you can contact us at patientsfirst@findmecure.com.
TrialHub is a feasibility intelligence platform that supports feasibility, startup, patient recruitment and clinical project managers with a 360-degree overview of the clinical research landscape in order to plan successful and on-time clinical trials.
TrialHub does this by aggregating thousands of data sources in real-time and combining them with local experts’ insights.
The data provided in this article is gathered on the 17th of December 2020 and is based on 17 clinical trial registries (clinicaltrials.gov, eudract.ema.europa.eu etc.) and analytics about thousands of clinical trials. Our platform is real-time so the data is dynamic.
If you want to get an up-to-date and/or customized feasibility for IBD or need an assessment about a different indication or TA, please contact us at patientsfirst@findmecure.com

